Introduction
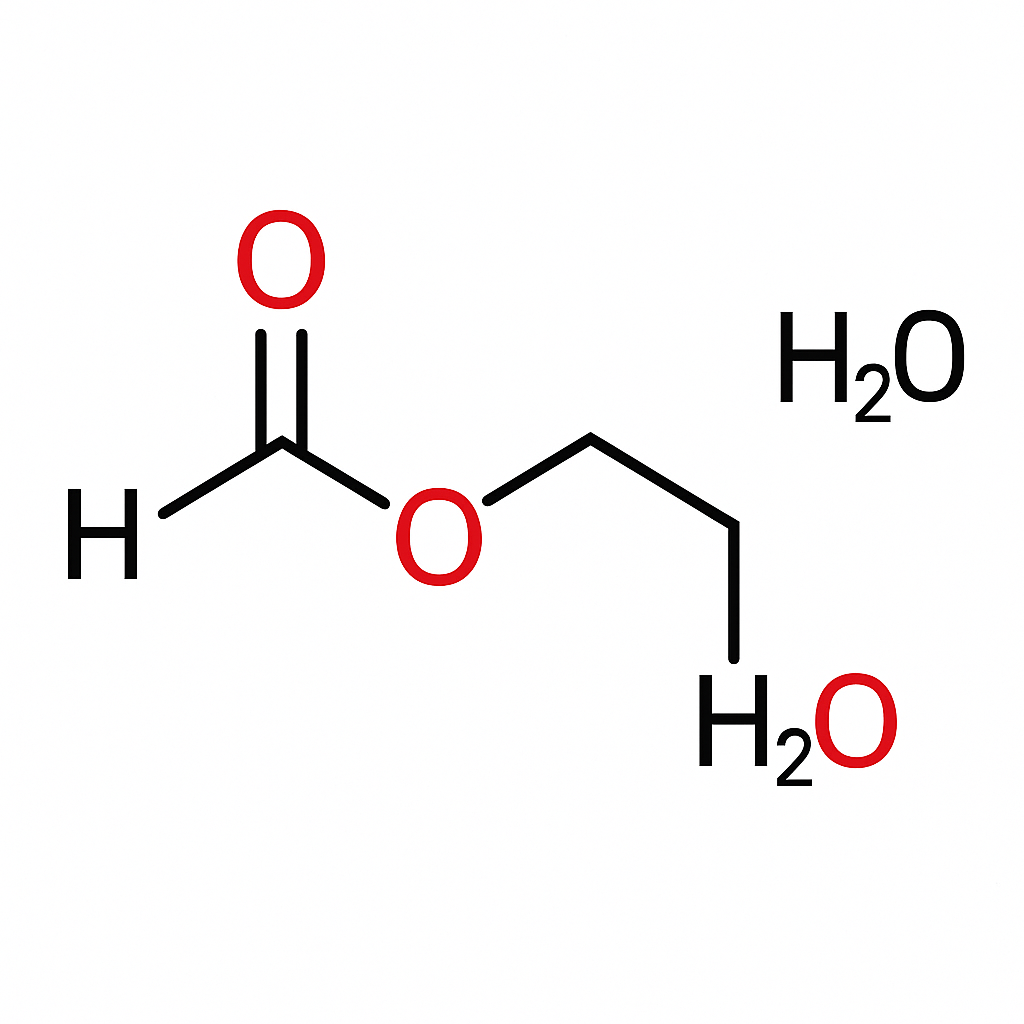
Ever stared at a chemical formula and thought, “Wait, what on earth is that?” You’re not alone! The formula HCOOCH CH2 H2O might look like a random jumble of letters and numbers, but beneath that cryptic shell lies a story about organic chemistry, molecular reactions, and the subtle dance of atoms that make up so much of what we see—and don’t see—around us.
Chemistry, at its heart, is a storyteller. It tells us how the smallest things—atoms—come together to create big, beautiful, and sometimes bizarre structures. And HCOOCH CH2 H2O? Well, that’s no exception. It’s a glimpse into how formic acid derivatives, water molecules, and organic groups interact to shape the reactions that fuel life, industry, and innovation.
Let’s roll up our sleeves and dive into this wonder molecule—step by step, bond by bond!
Understanding the Basics of HCOOCH CH2 H2O
So, what exactly is HCOOCH CH2 H2O? Let’s break it down before our brains start bubbling like a chemistry lab experiment gone wild.
At first glance, this formula seems odd—it combines fragments that resemble formic acid (HCOOH), methanol (CH3OH), and of course, water (H2O). When chemists see such patterns, they immediately think of esters, hydration reactions, and hydrolysis.
In short:
-
HCOOCH suggests a formate group (linked to formic acid).
-
CH2 represents a simple carbon-hydrogen chain.
-
H2O, the universal solvent, indicates a hydration process or reaction medium.
When combined in the right conditions, these can represent the intermediate steps in the formation or breakdown of esters—those sweet-smelling compounds that make perfumes, fruits, and flavorings so irresistible!
The Chemistry Behind the Formula
Chemistry, as every lab coat-wearing wizard will tell you, is all about interactions. The beauty of HCOOCH CH2 H2O lies in its ability to form and break bonds under mild conditions, revealing key insights into how organic reactions work.
Let’s simplify this:
1. Formation Through Esterification
The likely reaction could involve:
Formic acid (HCOOH) + Methanol (CH3OH) → Methyl formate (HCOOCH3) + H2O
Here’s where it gets juicy. If we strip this down to symbolic shorthand, it mirrors HCOOCH CH2 H2O. This hints at the esterification process—a cornerstone of organic chemistry that creates sweet-smelling compounds.
What happens here?
-
The carboxylic acid group (–COOH) reacts with the hydroxyl group (–OH) from an alcohol.
-
Water (H2O) is produced as a by-product.
-
The result? An ester—a compound with a pleasant odor and practical uses galore!
This simple-looking process powers industries from fragrance to biodiesel production.
2. Hydrolysis: The Reverse Reaction
Now, if you add water instead of removing it, you flip the script. The ester breaks down, forming the original acid and alcohol again. This process is called hydrolysis.
So essentially, HCOOCH CH2 H2O is a chemical snapshot of a reversible reaction—like catching a pendulum mid-swing!
Think of it this way:
-
Add water → break the bond → get acid + alcohol.
-
Remove water → form bond → get ester.
This dynamic balance forms the backbone of equilibrium chemistry, where reactions don’t just go one way—they dance back and forth.
Real-Life Applications of HCOOCH CH2 H2O Chemistry
You might be wondering, “Okay, but how does this chemistry stuff help me outside the lab?” Oh, you’d be surprised! Let’s look at where this kind of reaction shows up in everyday life.
1. Fragrance and Flavor Industry
Esterification reactions, similar to what we see in HCOOCH CH2 H2O, create compounds with fruity, floral aromas. The scent of pineapple, banana, and strawberry often comes from esters like methyl formate or ethyl acetate.
These reactions are controlled, repeated, and refined to give perfumes their characteristic notes or to flavor your favorite candies and beverages.
2. Biofuels and Green Energy
Ester reactions form the base for biodiesel production! When oils or fats react with alcohols, they produce fatty acid esters—the main components of biodiesel.
That’s right—chemistry like HCOOCH CH2 H2O isn’t just bookish science; it’s helping fuel vehicles sustainably.
3. Laboratory and Industrial Use
In labs, esters are used as solvents or reagents. Methyl formate—a compound similar in nature to HCOOCH CH2 H2O—is used to make formic acid and other chemicals.
Industrial chemists manipulate such molecules to produce plastics, pharmaceuticals, and cleaning agents.
The Curious Behavior of Water (H2O) in the Mix
Water seems so innocent, doesn’t it? Yet, in chemistry, it’s a troublemaker and a hero.
In the HCOOCH CH2 H2O reaction framework, water can either:
-
Help form compounds (through hydration).
-
Break them apart (through hydrolysis).
This dual nature gives it incredible power in both nature and industry. In biological systems, for instance, enzymes catalyze similar hydrolysis reactions daily—breaking down fats, proteins, and carbohydrates.
Without this delicate dance, life simply wouldn’t exist.
Why This Reaction Matters in Organic Chemistry
Alright, so we’ve established that HCOOCH CH2 H2O isn’t just random—it’s a symbolic representation of organic interplay. But why do chemists care so much about such reactions?
Here’s why:
-
It teaches reversibility.
-
Not all reactions are one-way streets. Understanding reversible systems helps scientists manipulate equilibrium for desired outcomes.
-
-
It connects to real-world synthesis.
-
Making perfumes, fuels, or drugs—all require mastering esterification and hydrolysis.
-
-
It’s foundational learning.
-
Every chemistry student meets this reaction early on—it’s the “hello world” of organic synthesis!
-
-
It reveals molecular relationships.
-
How acids, alcohols, and water relate is a key concept that underpins countless industrial processes.
-
The Fun Side of Chemistry
You might think chemistry is all formulas and explosions, but the story of HCOOCH CH2 H2O shows its creative side. Think of molecules as actors and reactions as their scripts. Depending on the environment—heat, catalyst, or concentration—the story changes.
When chemists add a catalyst (like sulfuric acid), the esterification speeds up dramatically. Remove heat, and hydrolysis might slow to a crawl. It’s a molecular drama with pace, twists, and an ever-changing plotline!
And speaking of drama—don’t you find it fascinating how adding a mere drop of water can flip the entire outcome of a reaction? Talk about a power move!
A Peek into Future Chemistry
With sustainable chemistry taking center stage, understanding reactions like HCOOCH CH2 H2O is more vital than ever. Researchers are now exploring:
-
Green catalysts to replace harmful acids.
-
Solvent-free reactions that reduce waste.
-
AI-based modeling to predict reaction pathways before experimentation.
The humble esterification reaction might just become a hero in eco-friendly chemistry, minimizing pollution while maximizing productivity.
Common Misconceptions About HCOOCH CH2 H2O
Let’s clear up a few things, shall we?
Misconception 1: It’s a random formula.
Nope! It actually represents a reaction or intermediate state involving formic acid, methanol, and water.
Misconception 2: It’s irrelevant outside labs.
Wrong again. From perfumes to biofuels, ester reactions are everywhere.
Misconception 3: It’s too complex for beginners.
Not at all! Once you see how acids, alcohols, and water interact, it’s surprisingly logical—almost poetic.
Quick Recap: The Magic Formula of HCOOCH CH2 H2O
Let’s summarize the main takeaways before we wrap up:
-
HCOOCH CH2 H2O symbolizes reactions involving ester formation or hydrolysis.
-
The formula reflects a balance between formic acid, methanol, and water.
-
Such reactions are central to organic chemistry, with practical applications in flavors, fuels, and pharmaceuticals.
-
Water plays both creator and destroyer—making and breaking bonds with equal finesse.
-
These principles pave the way for green chemistry innovations.
FAQs About HCOOCH CH2 H2O
1. What is HCOOCH CH2 H2O used for?
It’s not a single compound but a representation of an esterification or hydrolysis process used in the creation of esters like methyl formate.
2. Why is water (H2O) important in this reaction?
Because it determines the direction—adding it causes hydrolysis, while removing it encourages ester formation.
3. Is HCOOCH CH2 H2O dangerous?
The chemicals involved can be hazardous if misused, but under controlled lab conditions, they’re safe and commonly studied.
4. Can this reaction occur naturally?
Yes! Similar processes occur in biological systems during metabolism and enzyme activity.
5. How does this reaction connect to daily life?
From the scent in your shampoo to the fuel in your car, esterification reactions (like this one) power modern convenience.
Conclusion: The Hidden Wonder of Simple Chemistry
When you first see HCOOCH CH2 H2O, it may look like meaningless chemical gibberish—but as we’ve explored, it’s a microcosm of chemistry itself: the balance between creation and decomposition, simplicity and complexity, art and science.
From the sweet-smelling perfumes you adore to the biofuels driving the future, this humble reaction’s influence is everywhere. So, the next time someone says chemistry is boring, you can smile and say, “Have you ever met HCOOCH CH2 H2O?”